Rodríguez - López Laboratory
Advanced Electroanalysis for Energy Materials



Teaching is an activity that I enjoy very much and that complements my growth as a scholar. Beyond the transmission of knowledge, my teaching develops long-lasting collaborative and critical thinking skills in students, fostering their ability to solve problems and to carry out independent learning. Mentorship of undergraduates, graduates, and postdoctoral researchers in the classroom and in the laboratory has enriched my program by engaging them in topics they can relate to their learning, in identifying new research directions and potential collaborations, in writing proposals, and in getting involved in scientific outreach in the community. The activities I have developed help fulfill the University mission by training a new generation of scientists that are knowledgeable in state-of-the-art electrochemistry and that exercise outreach in energy sciences as a professional value. My overarching objective as an educator is to deliver an exceptional experience to all students capitalizing on the unique resources at UIUC. My teaching philosophy largely relies on active learning. In my classes, I emphasize methods for creative problem solving and for developing the ability to search for meaningful information, rather than only focusing on exam proficiency. During the COVID-19 pandemic I strongly adopted the use of small groups through zoom breakout rooms to perform group activities that strengthened lecture. In graduate classes I also deploy the Problem-Based Learning (PBL) system, in which I deliver unstructured projects to students like what they would encounter in research and stress the importance of formulating clear problem definitions and identifying the knowledge base needed to solve them. I improve my teaching by learning from students and by creating opportunities to engage them in research. Interacting with the ACS Student Chapter that I advise since 2013, and mentoring undergraduate researchers helped me understand the needs and expectations of our students. During the summer of 2020 I participated in the Illinois Online Teaching Academy, from which I learned the importance of empathy and the need to restructure my courses to better serve the academic interests of my students during the pandemic.
Course objectives: This course aims at assisting senior undergraduate, first- and second-year graduate students in their effort to obtain external grants and fellowships. Using the NSF GRFP competition as a template, the course provides students with guidance and information on all aspects of preparing grant applications. Each student is required to prepare a complete and competitive application package by a deadline. Submission of the package to NSF (or other) is encouraged but not required. Students grain practice in writing, succinctly explaining their proposed topic to a general scientific audience and using their critical thinking and self-reflection skills regarding their personal context to assemble a compelling package. Learning strategies: A focus of the course is to dramatically improve student’s ability to clearly communicate their objectives, their research plan, and the aspects that make them unique researchers and individuals. I encourage students to share their ideas in oral and written manner often and openly to ensure that others understand their content. To this end, the class takes on the form of a writing, figure design, and personal experience workshop so that all students can gather ideas to help them express their project successfully. Outcomes: I felt accomplished that for the NSF cycle corresponding to the class, there were 7 awardees and 6 honorable mentions in chemistry in UIUC, of which 6 and 3, respectively, took the course I taught.
Course objectives: This course deals with the fundamentals and applications of electrochemistry with strong emphasis in electroanalytical techniques. A physicochemical approach to the electrode/electrolyte interface is provided as a means of understanding reactivity and electroanalytical measurements. Chem 524 prepares students for: 1) gaining understanding on electrochemical tools and how to use them in research; 2) swiftly analyzing, understanding, and interpreting electrochemical results; 3) developing a critical knowledge of current and past literature in the field, and; 4) becoming acquainted with useful computational resources for electrochemistry. Learning strategies: Electrochemistry can be abstract and difficult to understand without the proper tools and exercises. To complement the lecture, one day of the week is dedicated to the visualization laboratory. This experience consists of a series of simulation activities and demonstrations in my laboratory. Activities include the exploration of circuit designs using chronoamperometric and voltametric techniques, the empirical exploration of Nernstian relationships and Tafel plots, the demonstration of the concept of a limiting current using microelectrodes, demonstrations of cyclic voltammetry and impedance spectroscopy using simple redox mediated systems, a visit to the laboratory to see the rotating disk electrode in action, and a demonstration of how simulations are built (students develop an excel spreadsheet simulator) and used (students receive code for implementing their own simulations) to understand electrochemical mechanisms. A final project is assigned, which consists of a problem-based learning activity focusing on a selected number of topics of interest to the student. These topics include photo-assisted electron transfer, bioelectrochemistry, synchrotron techniques for electrochemistry, batteries, and spectroelectrochemistry. These activities were praised by students, who saw in the visualization lab concrete applications of class contents. Students solving problems in small teams in Chem 524 during visualization lab days.
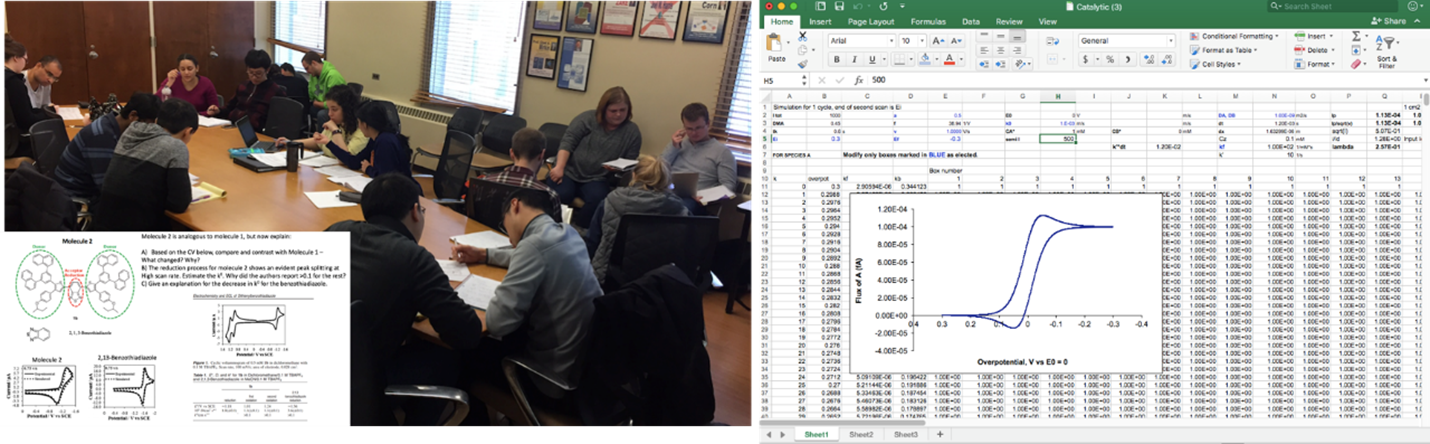
Course objectives: This course surveys the principles, instruments, and applications of physical materials characterization methods chemistry. The course addresses three major aspects of materials: Composition, Structure and Reactivity. Chem 588 prepares students for: 1) developing a critical knowledge of current literature and applications; becoming acquainted with useful resources for the surveyed techniques; solving complex problems and large tasks through teamwork, and; 4) taking charge of their own growth as a materials scientist it is not a passive course. Learning strategies: Graduate students will typically face complex challenges for which they need to develop a broad skillset that includes communication, leadership, critical thinking, and assessment of their own learning progress. Through problem-based learning (PBL), students solved broad scientific challenges in a collaborative environment. By addressing a multifaceted problem, students directed their own learning according to their interests. PBL activities help integrating the complex and foreign mix of instruments taught. The first PBL activity developed a protocol for analyzing a battery system in-situ. In the second PBL activity, students put together a mini-review on instrumental artifacts observed in diffraction and microscopy techniques. The third activity will consist of coupling multiple techniques for analyzing the functional aspect of a material. Surveys have revealed that students find PBL activities quite useful. Some PBL activities in Chem 588, showing a prompt, and the output from the students, showing how a compendium of artifacts was pieced together by the entire class:

Course objectives: This class is intended for developing skills in students that will be useful in all sub-disciplines of chemistry. The course introduces students into the practice of quantitative chemical analysis from the perspective of “wet chemistry” with the following objectives: 1) understanding the chemical aspects of statistical error and how it impacts measurement; 2) acquiring an in-depth knowledge of the algebra of chemical reactions; 3) applying this knowledge to solve general problems in acid/base chemistry, solubility, activity, complexometry, separations, and redox chemistry; 4) realizing the value of transducing chemical processes into electrical quantities, and; 5) realizing the value of analysis by light-matter interactions as in spectrophotometry. Learning strategies: This class is intended for developing skills in students that will be useful in all sub-disciplines of chemistry. The course introduces students into the practice of quantitative chemical analysis from the perspective of “wet chemistry” with the following objectives: 1) understanding the chemical aspects of statistical error and how it impacts measurement; 2) acquiring an in-depth knowledge of the algebra of chemical reactions; 3) applying this knowledge to solve general problems in acid/base chemistry, solubility, activity, complexometry, separations, and redox chemistry; 4) realizing the value of transducing chemical processes into electrical quantities, and; 5) realizing the value of analysis by light-matter interactions as in spectrophotometry. Joaquín using the lightboard.

Course objectives: This course introduces students to analytical measurements form the point of view of instrumentation, including the following objectives: 1) understanding modern instruments, including their parts, logic, and outlook for use; 2) understanding the limitations of measurement by exploring statistical error and noise; 3) acquiring an in-depth knowledge of components of instruments; 4) applying this knowledge to understand how optical, chromatographic, mass spectrometric, scanning-probe and electron methods work. Learning strategies: Chem 420 is an essential course for modern chemistry as it teaches students fundamentals of using instruments to perform chemical measurement, and instruments are everywhere! Unfortunately, it is often regarded as an information-intensive, boring, tedious course. In my first experience teaching it during Fall 2018, the first time I taught this course, I obtained my lowest post-tenure ICES evaluation. This experience made it clear to me that restructuring the course was imperative. Having an outstanding experience restructuring Chem 222 (see above), I decided to apply the same strategies, emphasizing the role of small-group discussions using zoom break rooms to allow students to experiment with the learning content. I designed a master powerpoint slide with instrument block elements that we used throughout the course to design instruments. Students discussed, and took design risks, and even generated questions that I had to consult with colleagues to fully address. They also appreciated our empathetic approach, which was nothing like what they had heard before about the course. I felt a profound sense of achievement in turning around the perception of this course. Sample of active learning activity used in Chem 420:
