Rodríguez - López Laboratory
Advanced Electroanalysis for Energy Materials



Identifying structure-activity correlations at electrochemical interfaces greatly benefit from simultaneously identifying materials properties AND local reactivity. Our combinations of Raman (and other laser based-methods) and scanning electrochemical microscopy (SECM) create new opportunities for elucidating total reactivity at the micro- and nanoscale.
Key results and exciting directions in our laboratory include:Electrochemical techniques generate information that is highly machine-friendly for artificial intelligence methods. Exploring new materials and new materials properties also greatly benefits from micro- and nano-scale characterization. Our group is pioneering approaches bolstered by small electrodes, integration with microfluidics, microfabrication, and integration with Python and robotics for addressing the materials challenges of the 21st century.
Key results and exciting directions in our laboratory include: The atomic thickness of graphene makes it an intriguing material for electrode design. Our group has pioneered studies of outer-sphere electron transfer, electrocatalysis, and
ion-insertion in ultrathin graphene electrodes by addressing a fundamental question: when do bulk and interface properties converge?
Key results and exciting directions in our laboratory include:
We create new quantitative imaging methods for energy storage and electrocatalysis using the scanning electrochemical microscope (SECM).
The outstanding versatility of this instrument to capture the reactivity gives us a unique advantage to design advanced materials and interfaces and to explore creative solutions to unresolved questions in interfacial chemistry.
In particular, the coupling of SECM with Raman spectroscopy has helped in the study of structure-reactivity relationship of various systems.
Key results and exciting directions in our laboratory include:
We take a deep-dive into the relationships between electrode structure and reactivity. By introducing groundbreaking analytical platforms, advanced single-site and surface-sensitive imaging methods, and time-resolved, in-situ, and chemically-resolved measurements create new and unique knowledge that informs new strategies for superior catalysts.
Key results and exciting directions in our laboratory include:
We created a new type of redox-flow battery that is based on size-exclusion: energy-storing redox polymers act as energy carriers, enabling the use of highly efficient
and inexpensive nanoporous membranes that have dramatically improved the performance of non-aqueous flow technologies.
Key results and exciting directions in our laboratory include:
We have highlighted our cutting-edge electrochemistry in several invited documents, opinion pieces, perspectives, and book chapters. We take pride in advancing, modernizing, and disseminating electroanalysis to ensure that the role of analytical chemistry remains strong in understanding the chemistry behind important developments in energy science.
 Book Chapter Scanning Electrochemical Microscopy. Chapter 16: Application to Batteries and Fuel Cells |  Book Chapter Encyclopedia of Electrochemistry. Chapter: Methods and Instrumentation in Energy Storage |  Book Chapter Batteries: Materials Principles and Characterization Methods. Chapter 9: SECM: A Versatile Tool for Inspecting the Reactivity of Battery Electrodes |  Publication #60 Advanced Electrochemical Analysis for Energy Storage Interfaces |  Publication #59 Electrocatalysis on Ultra-Thin 2D Electrodes: New Concepts and Prospects for Tailoring Reactivity |
 Publication #52 p>Prospects for Single-Site Interrogation Using In Situ Multimodal Electrochemical Scanning Probe Techniques |  Publication #47 Finding Harmony Between Ions and Electrons: New Tools and Concepts for Emerging Energy Storage Materials |  Publication #39 Redox-Active Polymers as Soluble Nanomaterials for Energy Storage | Publication #31 Emerging Scanning Probe Approaches to the Measurement of Ionic Reactivity at Energy Storage Materials < | 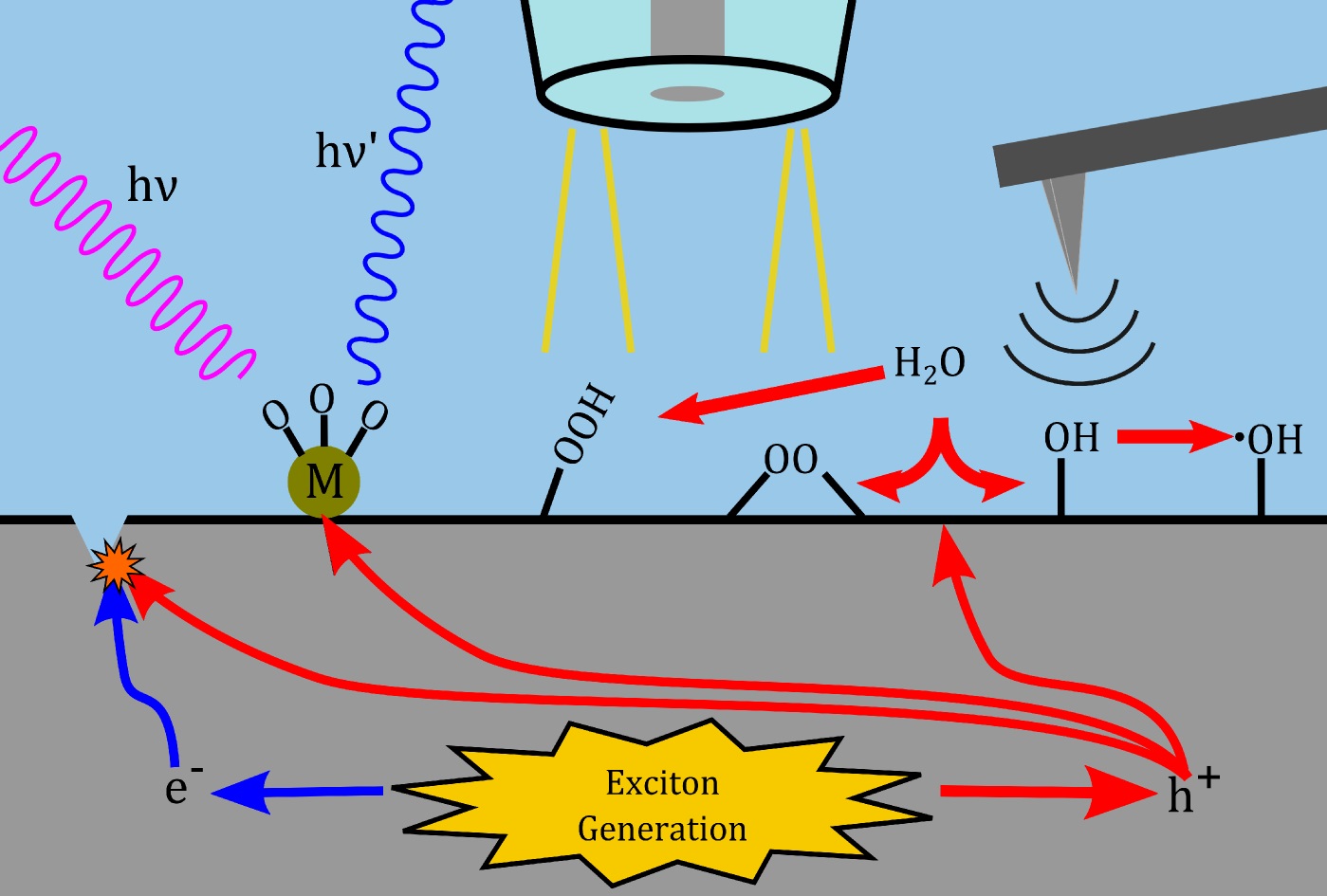 Publication #27 Emerging Techniques for the In Situ Analysis of Reaction Intermediates on Photo-Electrochemical Interfaces |
For a comprehensive list of publications, see our publications page and our Book Chapters section.
We are always interested in graduate students that are enthusiastic about electrochemistry, analysis, and energy materials. Please contact us regarding new postdoctoral opportunities as well!
Our group provides an exciting and motivating environment for scientific discovery and collaboration, both within researchers in the group and beyond. A strong emphasis will be placed in advising graduate students to think creatively, implement their ideas experimentally, and make use of computational modeling to test such ideas and independently generate more advanced ones.
See our group highlight in "Words of Wisdom", published in Chemical and Engineering News: